Chronic Lymphocytic Leukemia CLL Product release
Molecular Diagnostics
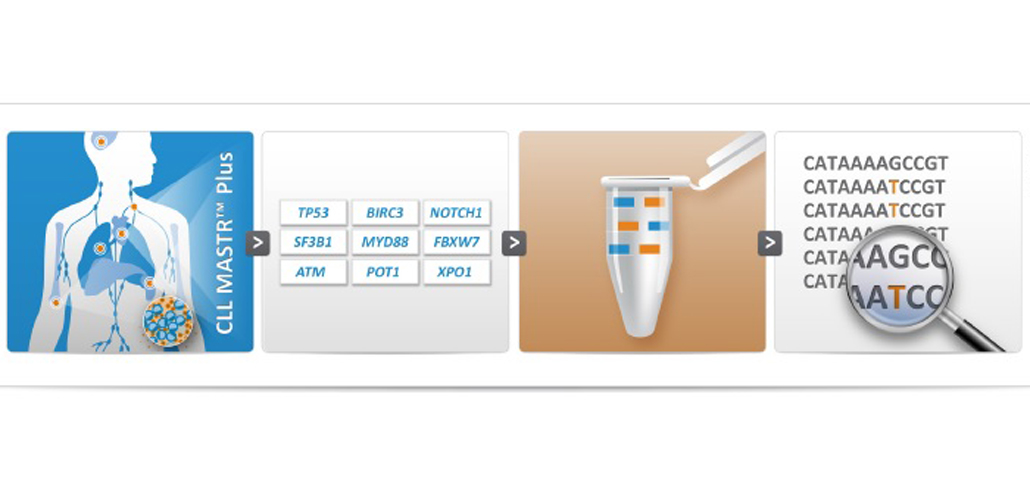
June 03, 2015Multiplicom is pleased to announce the release of the new CLL MASTR Plus, a RUO assay for identification of mutations in the coding regions of 9 genes related to CLL.
The CLL MASTR Plus is a molecular assay for the identification of both SNVs and CNAs in 9 selected genes (listed under the specifications tab) in individuals with indication of CLL.
Multiplicom’s CLL MASTR Plus assay is provided as a ready-to-use kit that offers robust performance with minimum hands-on time. All reagents for multiplex amplification of 251 unique amplicons (261-437 bp), ensuring complete coverage of all coding sequences in 6 PCR reactions are included.
The assay is compatible with all current Massively Parallel Sequencing (MPS) systems, providing the flexibility to choose your preferred method.