Revolutionary Next Generation IHC allows quantitative multiplexed images
March 12, 2015Labelled monoclonal antibodies analysed by mass spectrometry allow for quantitative multiplexing on intact tissue samples. This significant jump in the quantity of proteins detectable on a tissue sample is truly the next generation of IHC.
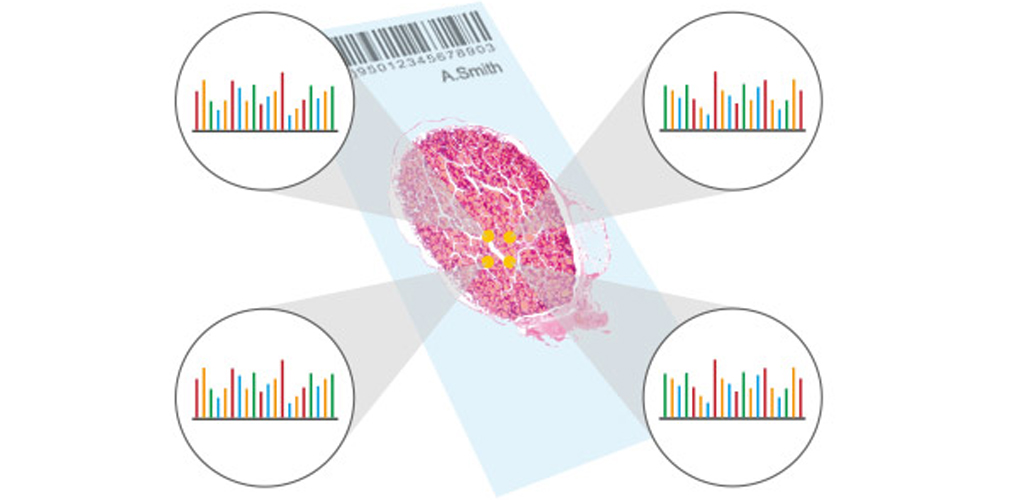
Recent advances in immunohistochemistry (IHC) techniques have improved their dynamic range by multiple orders of magnitude. This significant jump in the quantity of proteins detectable on a tissue sample is truly the next generation of IHC.
The dynamic range of IHC is 1 log and 2.5 logs for quantitative immunofluorescence (QIF), which measures the relative amount of a target protein in a tissue sample. Mass spectrometry IHC (MSIHC) can reach a value of 5 logs.
Currently there are limitations to IHC due to the restriction of detection techniques, loss of spatial resolution with chromogenic methods, loss of sensitivity with fluorescent methods, heterogeneity and variability in pre-processing of samples.
With the ability to allow multiplexed and directly quantitative imaging of tissue samples, this technique helps to overcome many of the current IHC limitations. It is indeed cutting edge technology for basic and clinical research.
The great advantage of this technique is that multiple monoclonal antibodies for the same protein are analyzed, revealing antibody specificity by directly comparing one antibody to another. It highlights the different specificity of the antibodies for different protein domains as a function of changes in the structure of the protein or post translational modifications.
The technique also has the advantage of allowing subtle changes in multiple proteins to be studied at once, due to the simultaneous use of a high number of heavy metal ions (high plexing capability). Finally, the multiplex advantage allows the use of antibodies against housekeeping proteins for reference and normalization, giving detailed pre-processing information and providing more reproducible and accurate IHC results. This opens the door to IHC as a more trustworthy clinical and diagnostic application.
It is important to note that for both methods the quality of the antibody is crucial. Poorly characterized or cross-reactive antibodies will give non-reproducible results because the interaction between antigen and antibody still has the primary role in the measurement of expression.
We offer RabMAb® products; a range of high quality rabbit monoclonal antibodies that complements this innovative technology. RabMAb products are ideal IHC reagents:
- Highly sensitive to the protein target due to the rabbit immune system's ability to develop highly specific antibodies
- As a monoclonal, highly specific to the target, with low background
- Always validated in IHC on a tissue microarray (TMA), tested on both positive and negative tissues
Additionally, we provide high quality rabbit monoclonal antibodies for anatomic pathology (IVD IHC usage).

dv55eRXDrydR9D1sib8D.jpg)
